Additional information
| Size | 100 points, 500 points, 2500 points, 5000 points, 10000 points |
|---|
$404.00 – $6,862.00
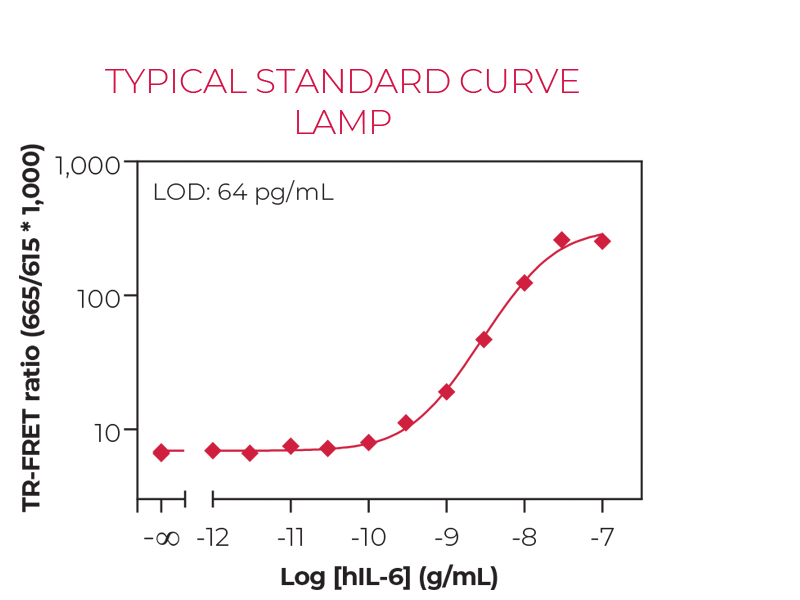
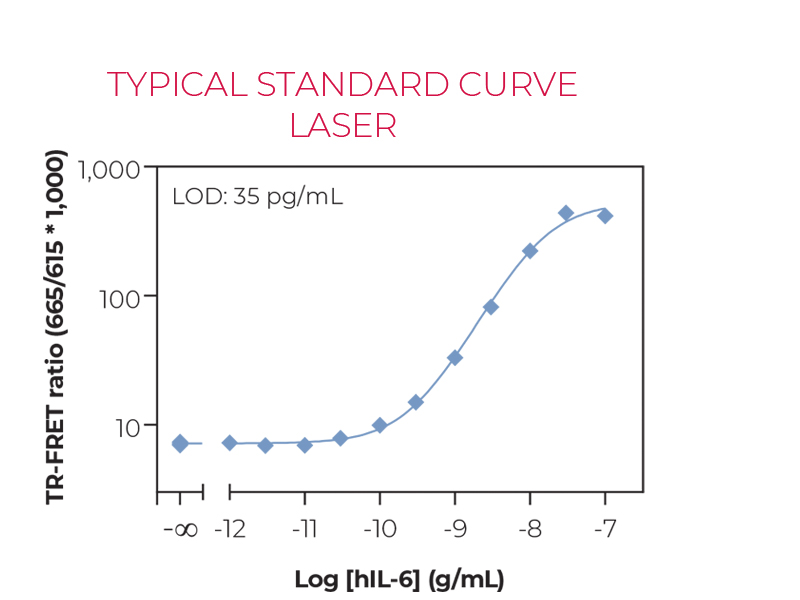
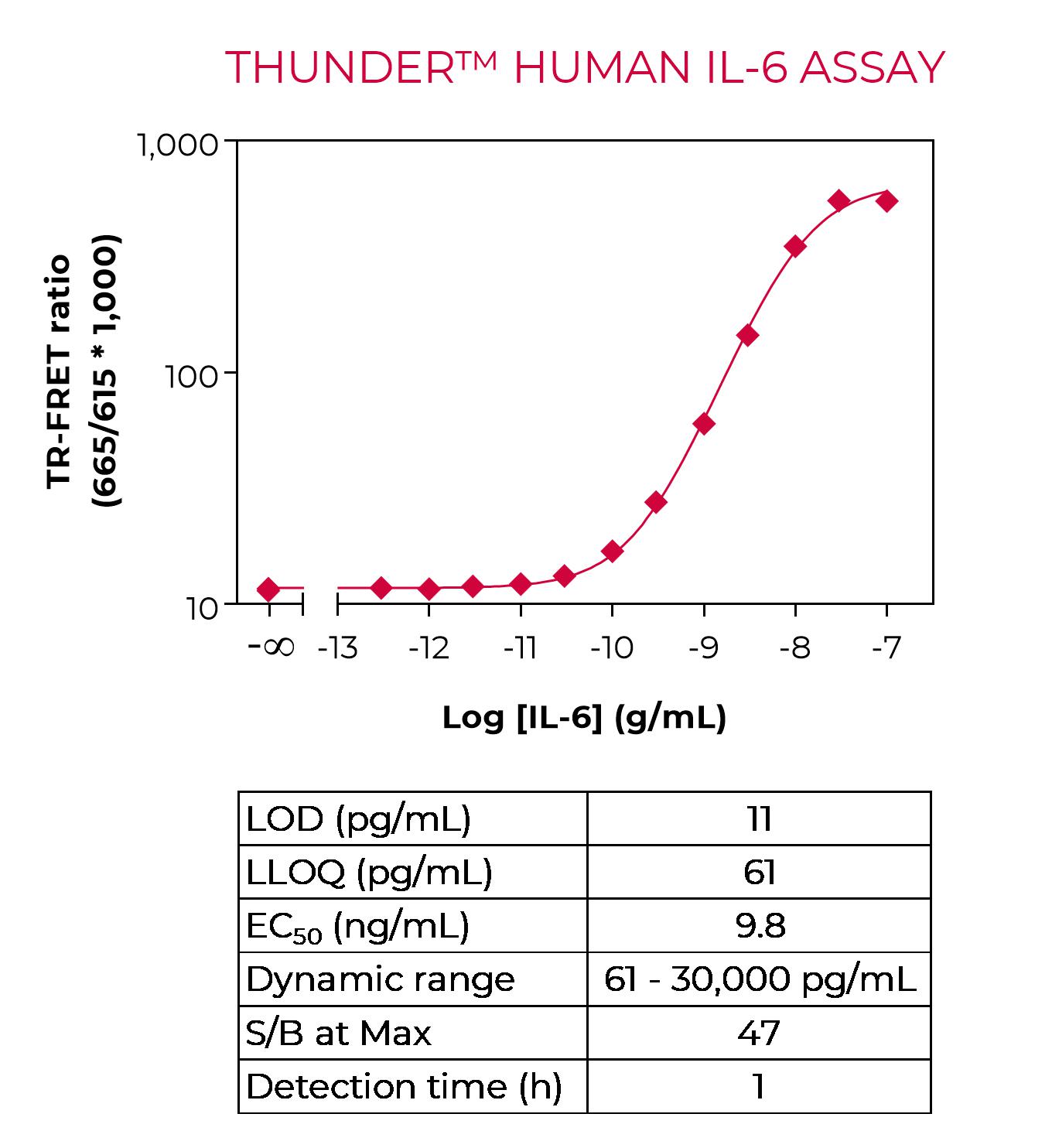
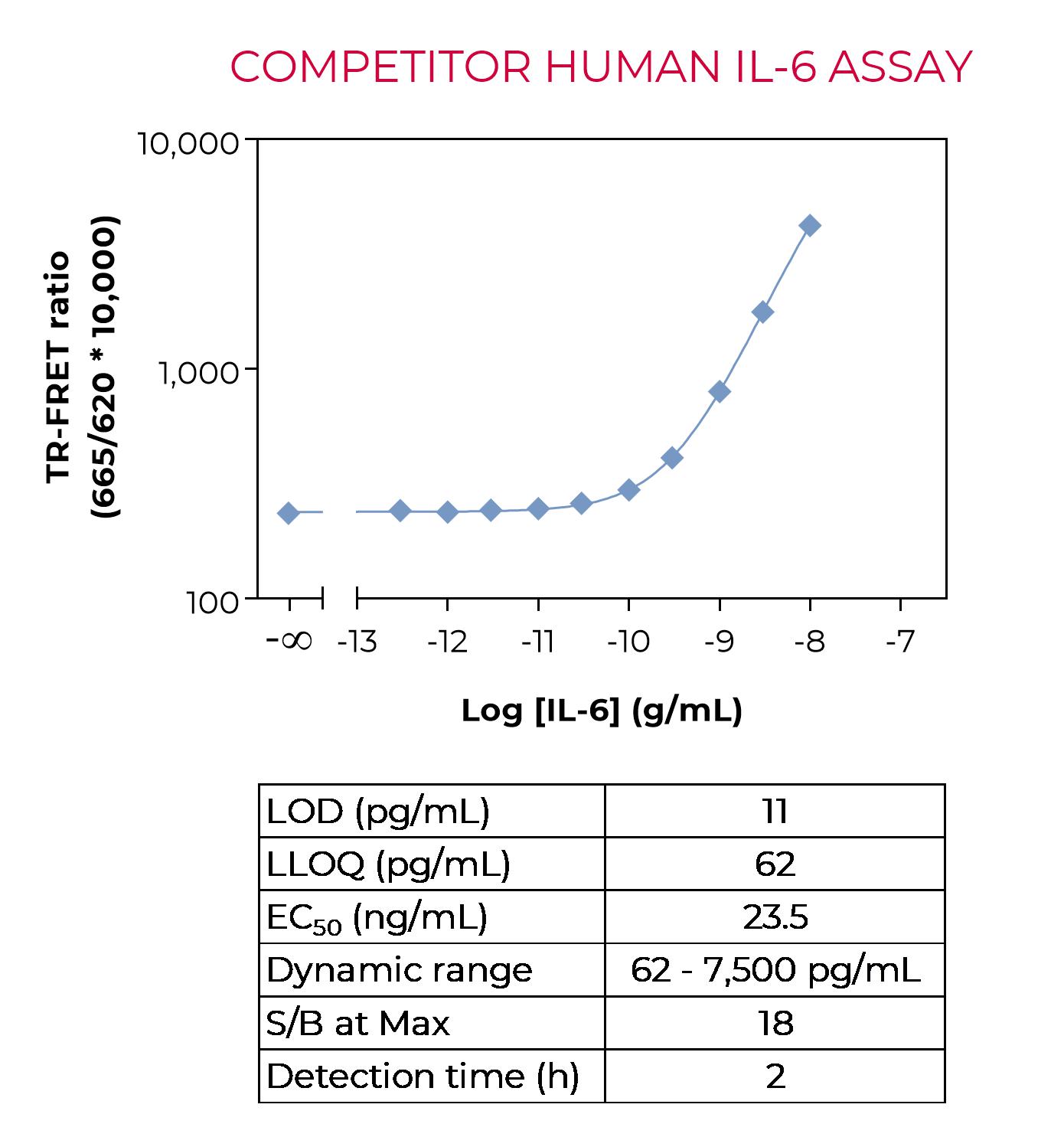
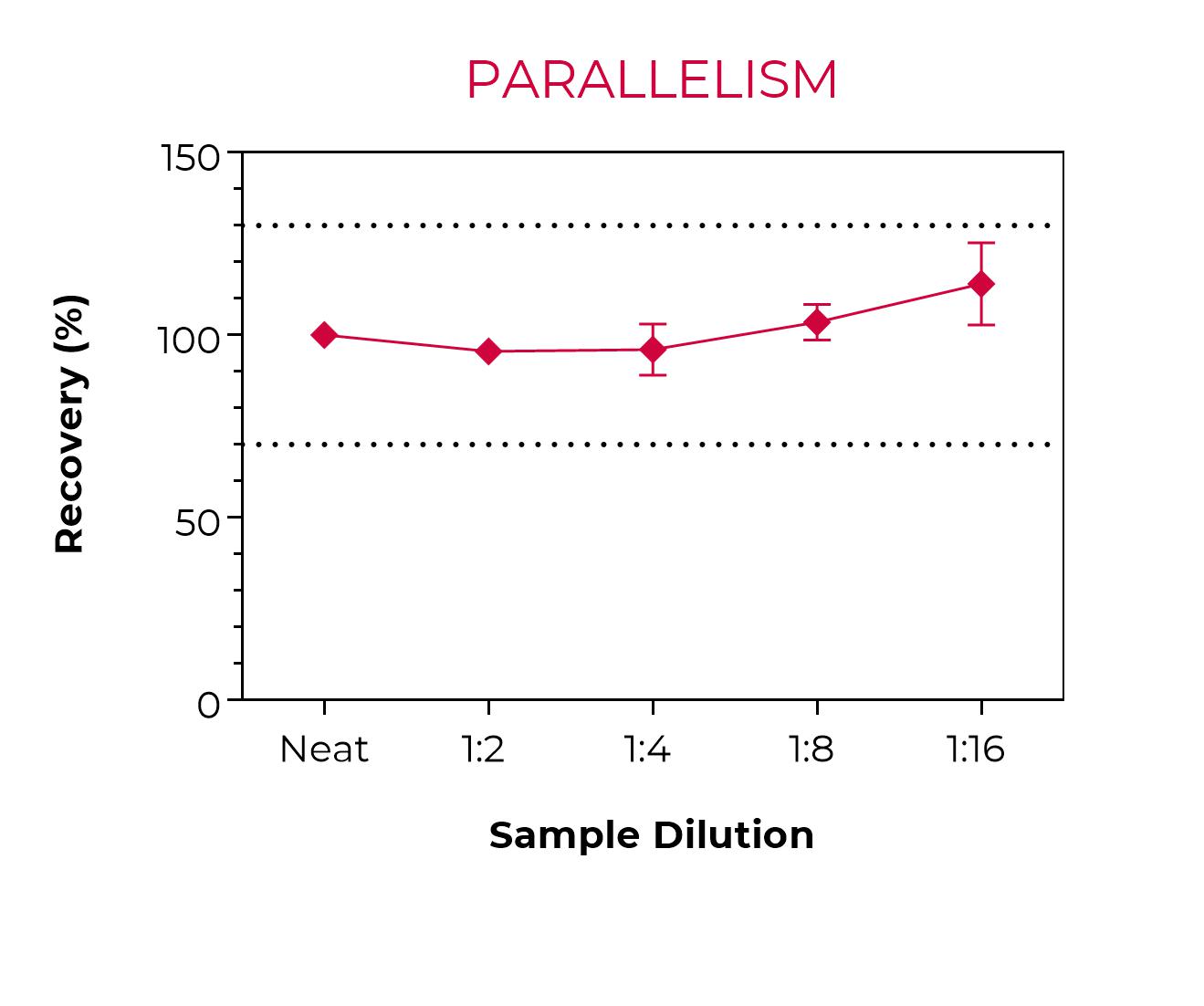
Sandwich TR-FRET immunoassay for the quantitative measurement of human IL-6 in cell culture supernatants. The kit does not include a recombinant human IL-6 standard, which must be purchased separately from ProSpec (catalog number CYT-213). A detailed assay protocol and full assay validation can be found in the Technical Data Sheet. BEST SELLER
Key features and benefits:
| Size | 100 points, 500 points, 2500 points, 5000 points, 10000 points |
|---|