Bioauxilium specializes in the design, development and manufacturing of ready-to-use TR-FRET assay kits that simplify laboratory workflow and accelerate drug discovery and life sciences research.

Bioauxilium is proud to announce the publication of an updated Application Note describing for the first time a comparison among three commercial TR-FRET assay platforms for measuring endogenous phosphoproteins in cellular lysates. The results of this head-to-head study demonstrate superior or comparable performance of THUNDER™ Cell Signaling Assays Kits to that of two existing TR‑FRET platforms while being more cost effective.
The availability of assays capable of measuring target-specific protein phosphorylation in a physiologically relevant cellular context is important for both basic research and drug discovery. Bioauxilium’s THUNDER™ is a new immunoassay platform based on an enhanced TR-FRET technology. The THUNDER™ Cell Signaling Assay Kits are designed to measure endogenous levels of specific intracellular phosphorylated proteins in cell lysates with high sensitivity, specificity, robustness and cost effectiveness (see Figure 1).
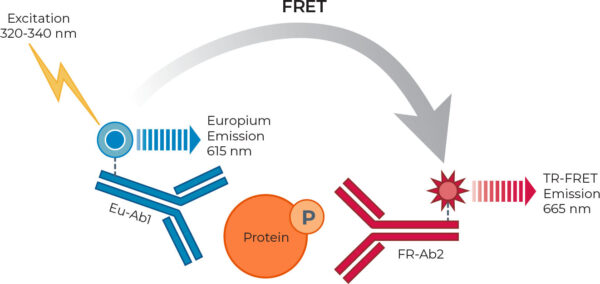
In this Application Note, we compared head-to-head the performance of THUNDER™ in 384-well plate format with two existing TR‑FRET technologies (Companies A and B) in measuring a panel of six phosphorylated proteins. The three TR‑FRET assay platforms were evaluated for their capacity to measure relative levels of phosphorylated 4EBP1 (T37/T46), AKT pan (S473), ERK1/2 (T202/Y204), p38αβγ (T180/Y182), SLP‑76 (S376), and STAT3 (Y705) in whole-cell lysates from cells treated with pathway-specific modulators. Of note, the THUNDER™ Phospho-SLP-76 and Phospho-STAT3 assay kits have been redeveloped using new antibody pairs to improve their performance.
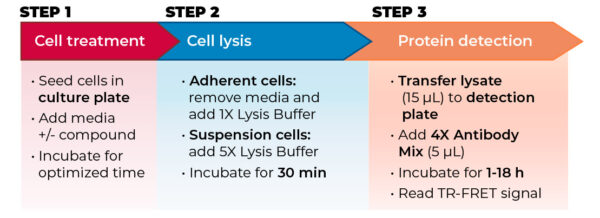
All assays were conducted according to each manufacturer’s protocols using the standard two-plate transfer protocol for each kit, whereby the cells are seeded, treated and lysed in a 96-well culture plate, and lysates are then transferred to a low-volume 384-well assay plate for protein detection (see Figure 2). Performance metrics were signal-to-background (S/B) ratio, IC50 and EC50, intra-assay variability (%CV), and stability of S/B ratio and IC50/EC50 following overnight incubation.
THUNDER™ assays showed the highest S/B ratios for phospho-AKT pan, phospho-ERK1/2, phospho-p38αβγ and phospho-STAT3. Comparable S/B ratios were obtained for THUNDER™ and
Company A phospho-4EBP1 assay as well as THUNDER™ and Company B phospho-SLP-76 (S376) assay. Company B exhibited the lowest S/B ratios for phospho-AKT pan, phospho-ERK1/2 and
phospho- STAT3. Data obtained for phospho-AKT pan is shown in Figure 3 below.
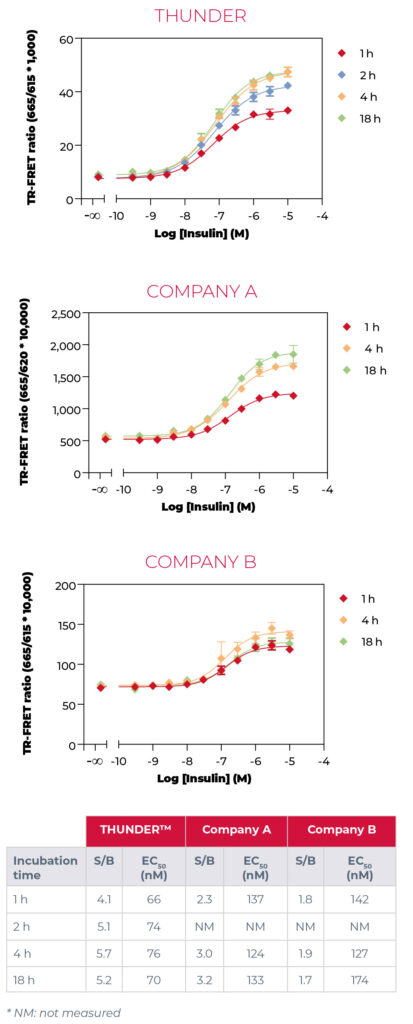
The three TR-FRET technologies exhibited comparable inter-well variability (typical %CV <8%) and sensitivity (IC50 and EC50 values) for all six phosphorylated proteins tested. In addition, all technologies tolerated plate reading after overnight incubation. An assay cost analysis showed that THUNDER™ assay kits are more cost-effective than the other two commercial TR‑FRET technologies.
Collectively, the results of these head-to-head comparisons showed that all THUNDER™ assays tested provide either superior or comparable S/B ratios, and comparable assay pharmacology. In addition, THUNDER™ is more cost effective than the other two TR-FRET assay platforms.
These key advantages, combined with rigorous assay validation using cell lysates from stimulated/inhibited cells, and higher flexibility in terms of kit sizes and formats (detection of either phosphorylated, total or phosphorylated plus total proteins with the same kit), make the THUNDER™ assay platform an alternative of choice for monitoring cellular protein phosphorylation.
See Application Note for more information.