
Bioauxilium specializes in the design, development and manufacturing of ready-to-use TR-FRET assay kits that simplify laboratory workflow and accelerate drug discovery and life sciences research.
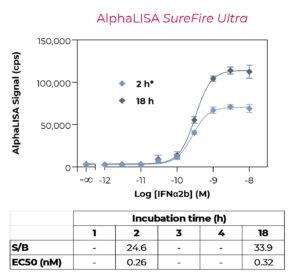
It’s no longer a secret that BioAuxilium’s THUNDER® is revolutionizing the market for drug discovery reagents by providing high-quality TR-FRET assay kits and reagents at affordable prices to the biomedical science community. The THUNDER® TR-FRET Cell Signaling Assay Kits are highly validated cell-based assay kits that enable the profiling of key cell signaling pathways. Recently, we have launched a panel of “High Performance” Phospho and Total STAT assays that exhibit improved sensitivity. In a new Application Note, we describe a head-to-head assessment of THUNDER®, HTRF®, and AlphaLISA™ SureFire® Ultra™ assays for the detection of phosphorylated STAT3 (Y705) in lysates from HeLa cells stimulated with interferon alpha-2b. The results show that the THUNDER High Performance Phospho-STAT3 assay outperformed HTRF and matched SureFire in terms of assay sensitivity while being simpler, faster, and more cost-effective.
We compared head-to-head the performance of THUNDER High Performance Phospho-STAT3 (Y705) assay in 384-well plate format with two other homogeneous assay platforms commonly used for drug discovery: HTRF and AlphaLISA SureFire Ultra.
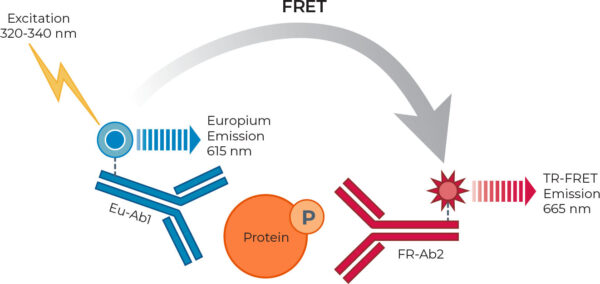
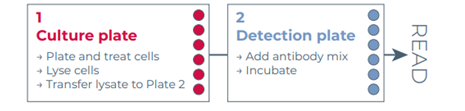
The three homogeneous assay platforms are based on the sandwich immunoassay principle. The assay principle of THUNDER is shown in Figure 1. All assays were conducted according to each manufacturer’s protocols using the standard two-plate transfer protocol for each kit, whereby the cells are seeded, treated and lysed in a 96-well culture plate, and lysates are then transferred to a low-volume 384-well assay plate for protein detection (see Figure 2). Performance metrics were signal-to-background (S/B) ratio, EC50, intra-assay variability (%CV), and stability of S/B ratio and EC50 value over time, ease of use, time-to-results and cost.
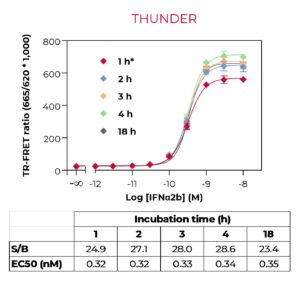
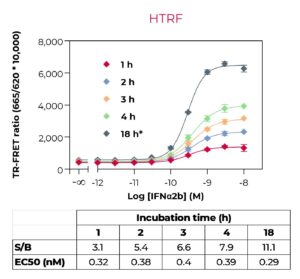
These key advantages, combined with rigorous assay validation using cell lysates from stimulated/inhibited cells, and higher flexibility in terms of kit sizes and formats (detection of either phosphorylated, total or phosphorylated plus total proteins with the same kit), make the THUNDER assay platform an alternative of choice for monitoring cellular protein phosphorylation.
See the Application Note for more information.