Bioauxilium specializes in the design, development and manufacturing of ready-to-use TR-FRET assay kits that simplify laboratory workflow and accelerate drug discovery and life sciences research.
An increasing number of pharma and biotech companies, as well as CROs and academic laboratories, are adopting BioAuxilium’s THUNDER® TR-FRET assay platform to quantify key human cytokines and chemokines in cell supernatants. Based on customer feedback, there are 3 key reasons.
Reason #1: High performance assays
THUNDER® Human Cytokine Assay Kits are designed to measure key human cytokines and chemokines in cell culture supernatants using simple, rapid, sensitive, and robust immunoassays based on the homogeneous (wash-free) THUNDER® TR-FRET technology. The assays are optimized to ensure high signal, low background, and the best sensitivity possible. All assays are based on the two-site sandwich immunoassay principle in which two highly specific antibodies are used to detect a target cytokine/chemokine.
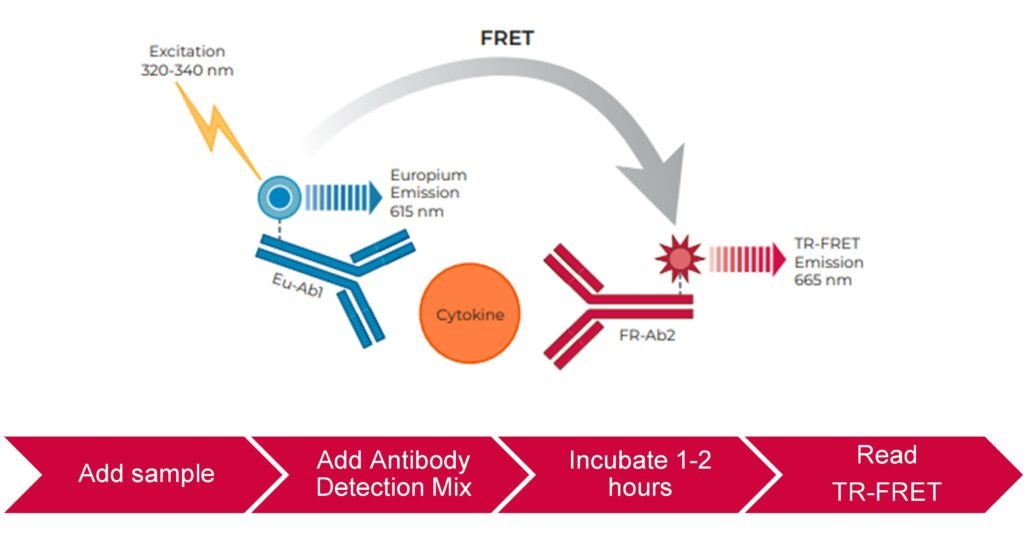
Figure 1: THUNDER™ TR-FRET assay principle and workflow.
Multiple steps are taken during assay development to ensure that all THUNDER™ Human Cytokine Assay Kits will provide superior performance without the need for further assay optimization by the user. These include:
In addition, the add-incubate-read protocol of the immunoassay platform, combined with its compatibility with all TR-FRET compatible microplate readers, facilitates streamlined workflows and rapid data generation. These assays are suitable for both drug discovery and basic research applications.
Table 1: Outstanding performance of selected THUNDER™ Human Cytokine Assays on the PHERAstar® FSX microplate reader.
| Analyte | Assay parameter |
PHERAstar FSX |
| IFNγ | LOD (pg/mL) |
6 |
| LLOQ (pg/mL) |
33 |
|
| S/B |
15 |
|
| IL-1β | LOD (pg/mL) |
1 |
| LLOQ (pg/mL) |
7 |
|
| S/B |
23 |
|
| IL-12p40 | LOD (pg/mL) |
3 |
| LLOQ (pg/mL) |
19 |
|
| S/B |
22 |
|
| TNFα | LOD (pg/mL) |
3 |
| LLOQ (pg/mL) |
41 |
|
| S/B |
57 |
|
| CCL2 | LOD (pg/mL) |
3 |
| LLOQ (pg/mL) |
17 |
|
| S/B |
17 |
Reason #2: Reliable assays
Another hallmark of the THUNDER™ Cytokine Assays is that they are fully validated to ensure they deliver the expected sensitivity, precision, accuracy, and stability. Validation testing includes:
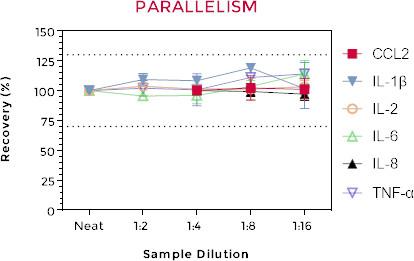
Figure 2: Cell culture supernatants from stimulated cells were serially diluted with culture medium and assayed for six human cytokines. Recovery values between 86-124% of the neat sample demonstrate assay parallelism.
These validation tests are performed by two operators to ensure that the assay will be reproducible both well-to-well, day-to-day, and lot-to-lot. We have a full transparency policy: all data obtained from validation testing are provided in the product data sheets so that the user can make an informed decision.
In addition, new lots are required to meet stringent manufacturing and quality control specifications to ensure that they provide the highest levels of performance and consistency. All lots are tested and compared to previous lots to ensure low assay background, a consistent standard curve and sensitivity, and a broad dynamic range.
Reason #3: Flexibility and affordability
These TR-FRET cytokine immunoassay kits are the most cost-effective on the market. They are available in five sizes for higher flexibility: 100, 500, 2500, 5000, and 10000 assay points. Bulk orders and lot reservation are available on request. Products are always in stock ensuring rapid delivery and are backed by an outstanding technical support.
In short, choosing THUNDER™ Cytokine TR-FRET Assay Kits allows scientists to generate reproducible and consistent data, while saving time and money. Can you afford not to try THUNDER™?